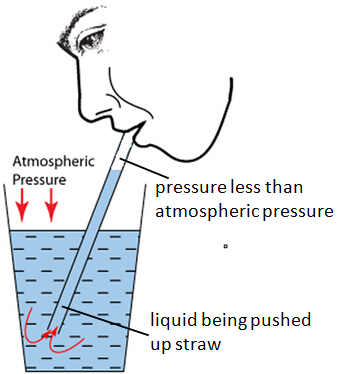
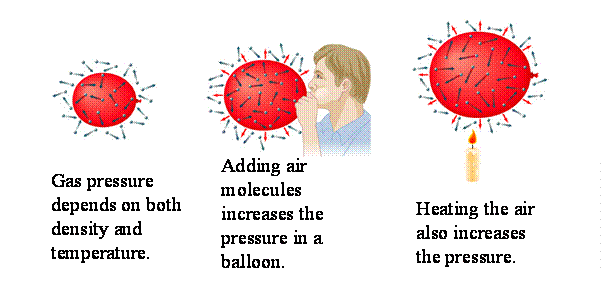
Last week, we had done an experiment with temperature using water and alcohol. Two tubes, one of water, the other of alcohol, were placed in one of the beakers on top of some kind of heater. We were to mark the tubes of wherever the liquid reached after the thermometer increased by 10 degrees Fahrenheit each time. Immediately, we noticed that the alcohol rose faster than the water, even though they were at the same exact temperature. Later, we figured out that was because that were both composed of different substances, which increased and decreased at different speeds. That wasn't the only experiment we attempted that day. We had two beakers, one filled with cold water and the other with hot, and placed a few drops of dye in each one. The hot beaker had red food coloring and when the food coloring entered the water, everyone could see how fast the red expanded through the water and finally blended within a few minutes. That's, because the particles were moving faster and when they move faster they are able to expand at a high rate. When we placed the blue food coloring in the cold water that was refrigerated the night before, however, it slowly spread out through the water. After five minutes, it still barely even expanded unlike the red food coloring that was already fully blended. We figured this was because the cold particles moved slower. The slow the particles moved/collided, the slower it would take for a substance to combine with another. We, also, learned about the different types of thermometers, how they worked, who invented them, and if they were still used today. We learned a lot that day, though it was centered all around temperature, like how substances rise and fall at different rates, how food coloring in hot water expands faster than if it was in cold, and all about thermometers and how they're important to us.